Pillar 1: Total body PET for Cell Tracking in Cell-based Therapies
Positron emission tomography (PET) is a technique that measures physiological function by looking at blood flow, metabolism, neurotransmitters, and radiolabelled drugs. Total-body PET (TBP) refers to a scanner that encompasses the entire body within the field of view and allows imaging of all the tissues and organs of the body simultaneously.
TBP is game-changing, it will greatly increase up to 40x sensitivity compared to conventional PET. This will lead to faster scans with much lower tracer doses, enabling new applications where radiation dose is currently limiting, for example, paediatric and perinatal applications. Additionally, it will allow deep molecular characterisation of multiple metabolic processes and gene expressions, in health and disease and as a function of ageing. Patients are currently scanned with only one tracer (typically [18F]FDG) on PET, whereas TBP will enable multiple radiotracers to be imaged, each revealing different molecular processes: ‘multiplexed PET’.
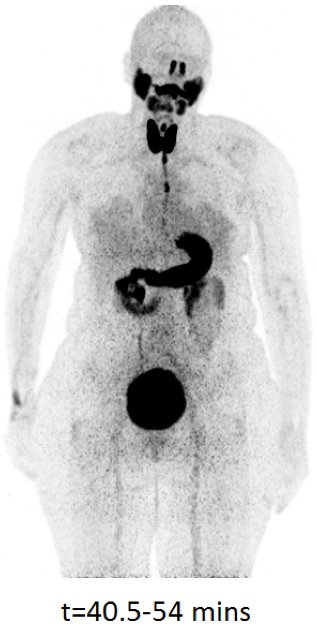
TBP will also be highly advantageous for cell tracking in the growing number of trials of cell-based therapy, for example, mesenchymal stem cells and CAR T-cells. Cell tracking is essential in this context to determine where administered cells go, how long they survive, and to what extent they may replicate. TBP will allow more prolonged scanning (allowing patients to be followed for weeks instead of a day or two) and/or lower cellular radiation doses than conventional radionuclide imaging.
In the current Centre the aim is to demonstrate the value of this approach by developing new dedicated radiotracers and methodology. Progress so far towards this goal includes the following developments:
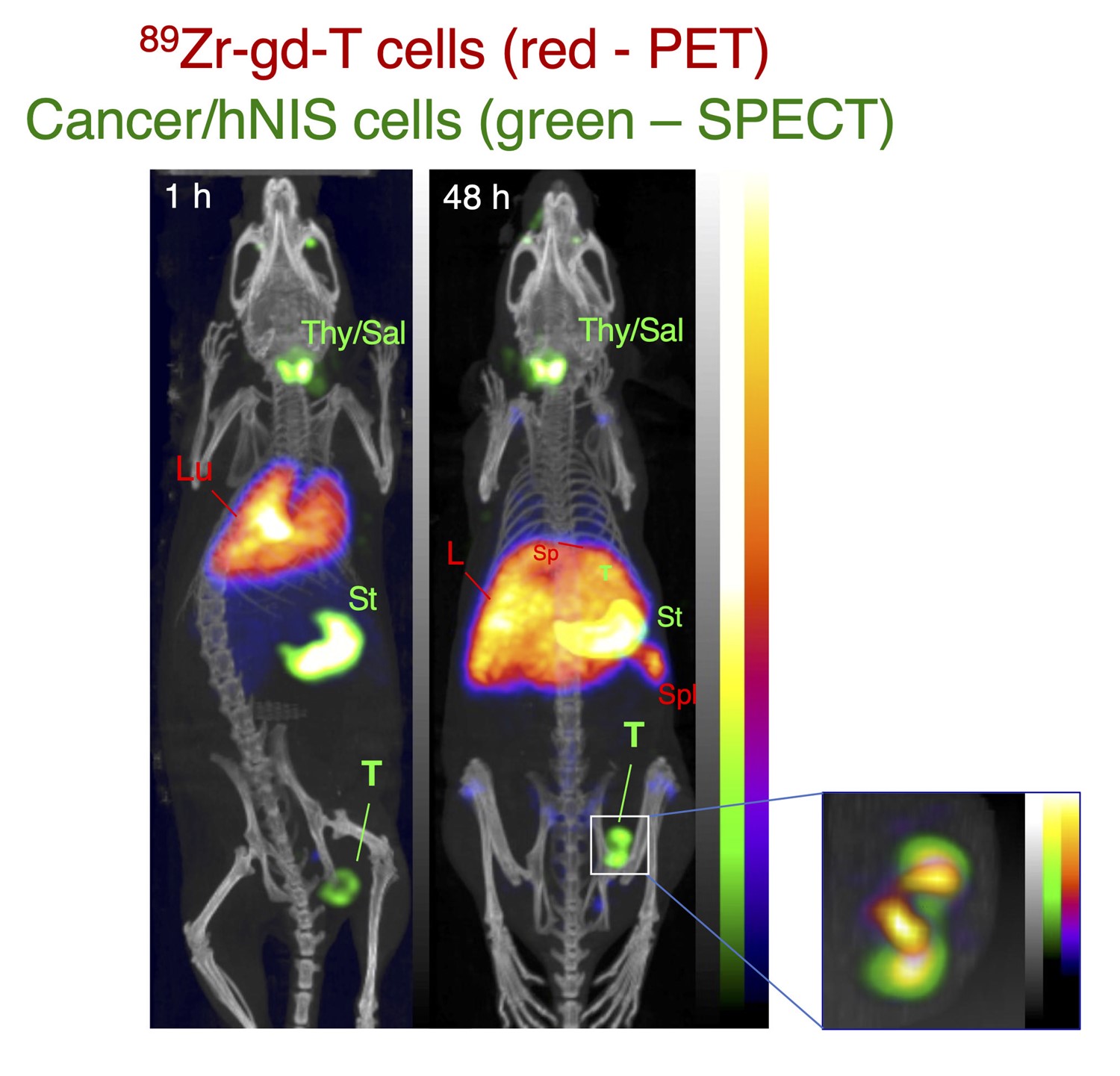
- The first long half-life, clinically translatable universal method for radiolabelling cells [1] and liposomal medicines [2] with zirconium-89 (half-life 78 h) – [89Zr]Zr-oxinate [3].
- A patented [4] kit-based formulation for simple, user-friendly application of the method, [5] demonstrated using the example of human leukocytes and preclinical studies [6].
- Reporter gene (for example, sodium/iodide symporter) expressing cell lines [7] and novel PET tracers ([18F]tetrafluoroborate,[8] [18F]fluorosulfate [9]) to image them longitudinally.
Several challenges remain to implement multiplexed PET and TBP cell tracking. Current work includes:
- Novel image acquisition and reconstruction methods for multiplexed PET and cell tracking.
- Improved design, diversity and versatility of positron-emitting cell labelling agents.
- Methodology for use of PET to quantify cell numbers spatiotemporally in the body.
- Understanding of the radiobiological effects of cell radiolabelling with positron emitters.
- First-in-patient conventional PET cell tracking studies within cell-based therapy trials.
The outcomes will complement new radiopharmaceutical chemistry for short half-life radiotracers specifically for multiplexed PET and cell tracking applications, developed within the parallel EPSRC MITHRAS Programme to underpin a new paradigm for enhanced imaging of multiple interacting molecular characteristics of disease, creating a holistic, systems-medicine tool for exploring human biology and characterising multifactorial disease.
Finally, it is a strategic aim of King’s and its partner NHS Trusts to host a national TBP facility, supported by King’s uniquely comprehensive clinical and preclinical radiochemistry and molecular imaging teams and facilities.

References
- Charoenphun, P. et al. (2015) ‘[89Zr]Oxinate4 for long-term in vivo cell tracking by positron emission tomography’, European Journal of Nuclear Medicine and Molecular Imaging, 42, pp. 278–287. doi: 10.1007/s00259-014-2945-x.
- Edmonds, S. et al. (2016) ‘Exploiting the Metal-Chelating Properties of the Drug Cargo for In Vivo Positron Emission Tomography Imaging of Liposomal Nanomedicines’, ACS Nano, 10(11), pp. 10294–10307. doi: 10.1021/acsnano.6b05935.
- Ferris, T. J. et al. (2014) ‘Synthesis and Characterisation of Zirconium Complexes for Cell Tracking with Zr-89 by Positron Emission Tomography’, Dalton transactions (Cambridge, England: 2003), 43(39), pp. 14851–14857. doi: 10.1039/c4dt01928h.
- UK pat app 2009512.1 Jun 2020
- Man, F. et al. (2020) ‘A kit formulation for the preparation of [89Zr]Zr(oxinate)4 for PET cell tracking: White blood cell labelling and comparison with [111In]In(oxinate)3’, Nuclear medicine and biology, 90–91, pp. 31–40. doi: 10.1016/j.nucmedbio.2020.09.002.
- Man, F. et al. (2019) ‘In Vivo PET Tracking of 89Zr-Labeled Vγ9Vδ2 T Cells to Mouse Xenograft Breast Tumors Activated with Liposomal Alendronate’, Molecular Therapy, 27(1), pp. 219–229. doi: 10.1016/j.ymthe.2018.10.006.
- Diocou, S. et al. (2017) ‘[18F]tetrafluoroborate-PET/CT enables sensitive tumor and metastasis in vivo imaging in a sodium iodide symporter-expressing tumor model’, Scientific Reports, 7. doi: 10.1038/s41598-017-01044-4.
- Jauregui-Osoro, M. et al. (2010) ‘Synthesis and biological evaluation of [18F]tetrafluoroborate: a PET imaging agent for thyroid disease and reporter gene imaging of the sodium/iodide symporter’, European Journal of Nuclear Medicine and Molecular Imaging, 37(11), pp. 2108–2116. doi: 10.1007/s00259-010-1523-0.
- Khoshnevisan, A. et al. (2017) ‘18F-Fluorosulfate for PET Imaging of the Sodium–Iodide Symporter: Synthesis and Biologic Evaluation In Vitro and In Vivo’, Journal of Nuclear Medicine, 58(1), pp. 156–161. doi: 10.2967/jnumed.116.177519.
